Full spectrum or “spectral” flow cytometry is a recently developed technology that allows high-dimensional flow cytometric analyses of cells and particles in suspension. This technology has gained popularity because it helps to quickly detect a large number of antigens simultaneously in the format of analysis in one test tube.
In spectral flow cytometers, essentially all the emitted light from each fluorophore is captured by a series of many detectors, for each laser. Therefore, each fluorophore is technically measured using several dozens of detectors simultaneously. This allows the spectral flow cytometer to establish a “spectral fingerprint” for each fluorophore's emitted light.
Although spectral flow cytometry is a modern technology, it is already widely used in medicine. In 2020, Cytek received CE-IVD certification for the use of its spectral flow cytometers and reagents in medicine. The company already has 5 offices in the USA (headquartered in Fremont, California), China, Japan and the Netherlands. The list of installed instruments already has more than 2200 cytometers around the world. The most advanced Cytek Aurora cytometer contains 5 lasers (5L 16V-14B-10YG-8R-UV16), three scattering channels and 64 fluorescence channels.
.jpg)
Cytek's full-spectrum technology makes it possible to perform real-time unmixing in SpectroFlo software. This provides more fluorochrome selection and panel flexibility and allows users to quickly display experiment results and statistics. All this features creates an ideal solution for flow cytometry for deep immunoprofiling of 24 to 40 colors.
One such panel is a 25-color panel designed and optimized by Cytek scientists to provide a turnkey solution for identifying the major human immune subpopulations that play an important role in the innate and adaptive immune response in various diseases.
Cytek 25-Color Immunoprofiling Assay allows for the identification of helper T cells, cytotoxic T cells, B cells, NK cells, NKT cells, Basophils, Dendritic cells, ILCs, and monocytes in human peripheral blood mononuclear cells and in whole blood. The reagents in this kit help to distinguish different subsets of T, B, NK, NKT, DCs; including regulatory T cells, naïve T cells, activated T cells, memory T cells, effector T cells, naïve B cell, memory B cells, Early NK, Mature NK, Terminal NK, nonclassical and classical monocytes.
CYTEK® 25-COLOR IMMUNOPROFILING ASSAY KIT
.jpg)
EXAMPLES OF DATA
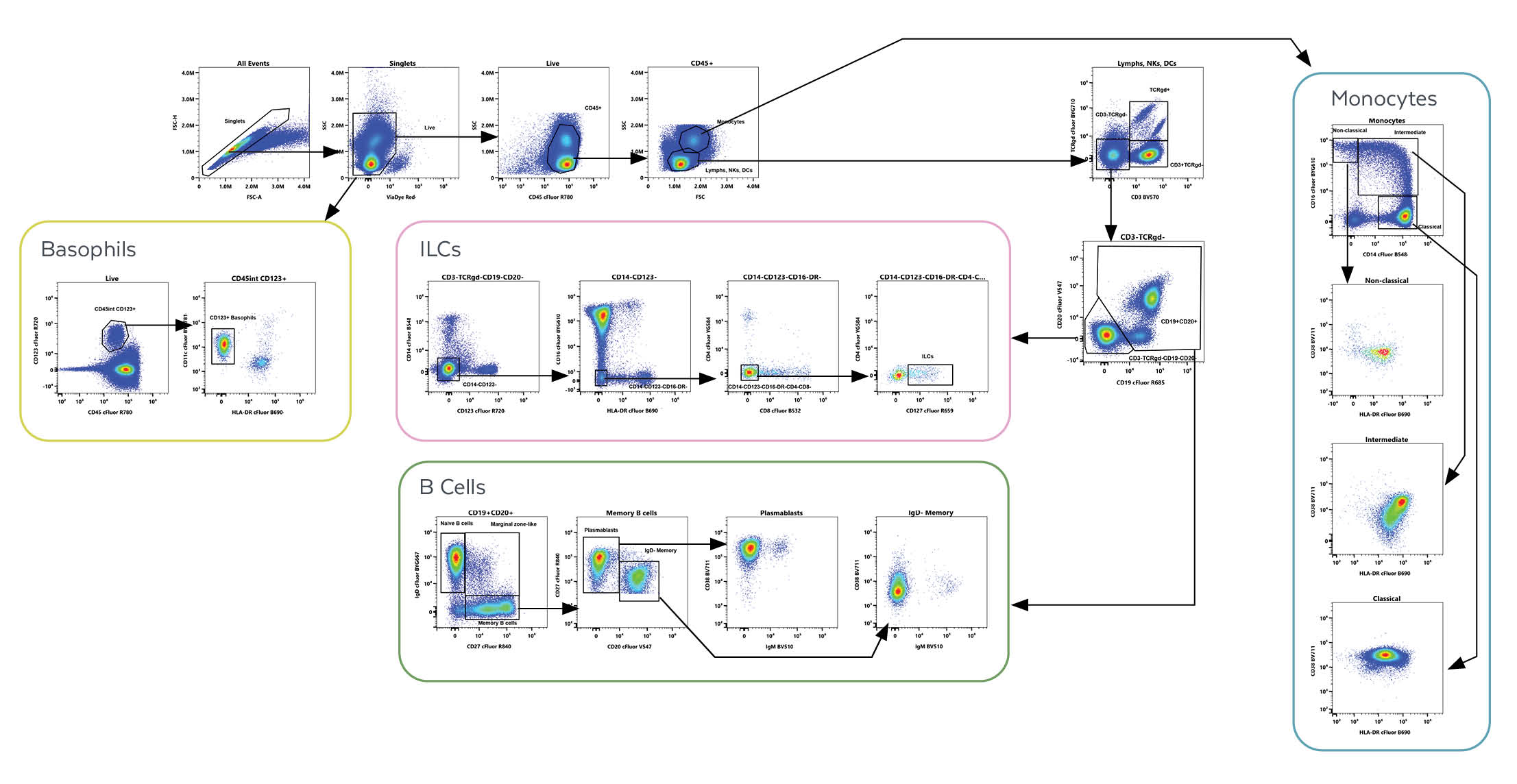
Next, consider an example of cell gating for population identification using the Cytek® 25-Color Immunoprofiling Assay Kit. Cell gating for population identification using the Cytek® 25-Color Immunoprofiling Assay Kit. Cells are first gated on singlets, viable cells, CD45+, and lymphocytes or monocytes based on scatter. Lymphocytes are divided into T cells, non-T cells, B cells, and ILCs. Monocytes are characterized into phenotypes by expression of CD14 and CD16. Basophils are classified in viable cells.
.jpg)
Within the T lymphocyte population, CD4+, CD8+, and CD4-CD8- T cells can be quantified and further segregated into naïve, memory, effector, and terminal effector subsets.
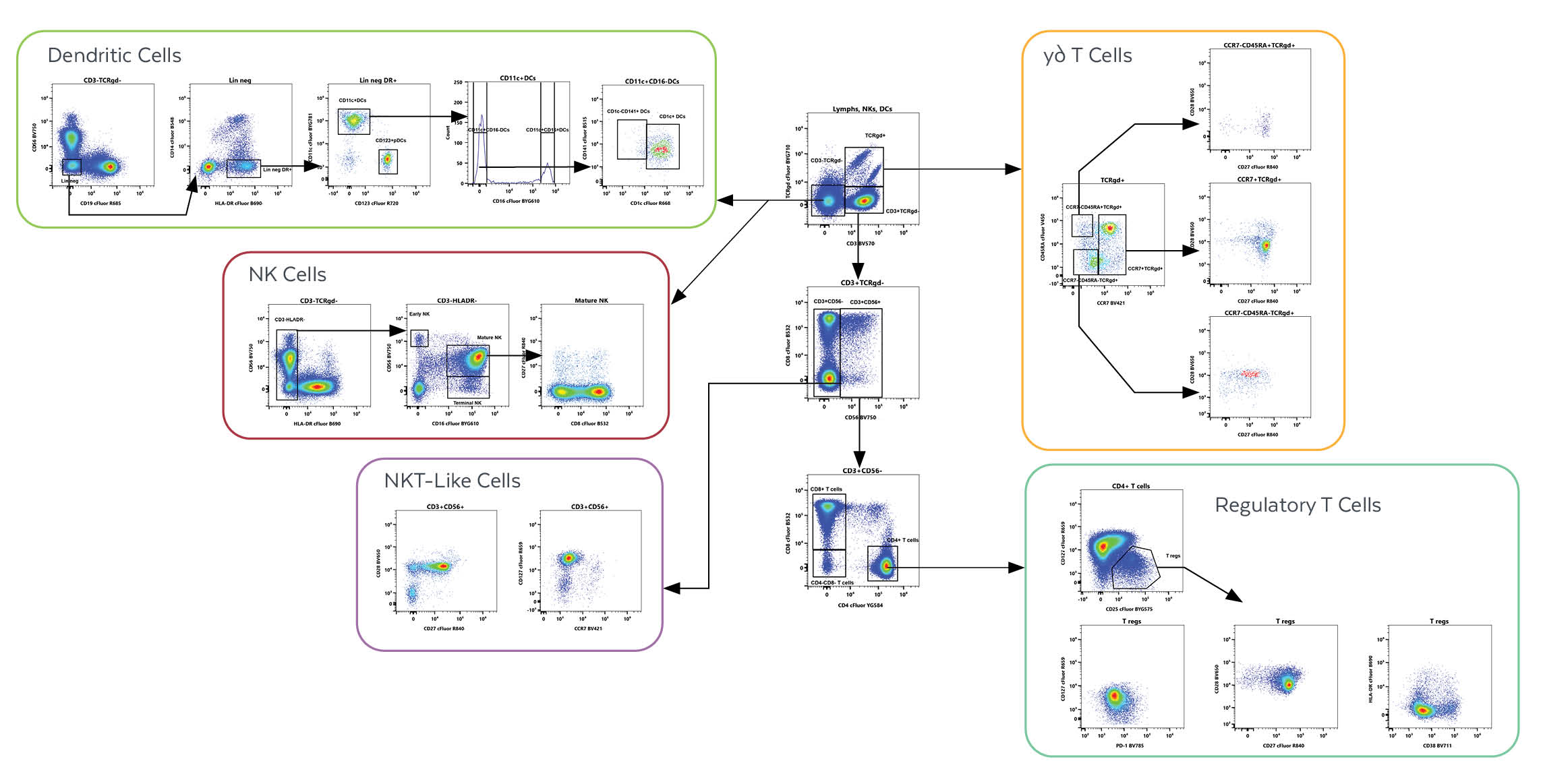
Additionally, identification and characterization of dendritic cells, NK cells, γδ T cells, NKT-like cells, and regulatory T cells are achievable using the Cytek® 25-Color Immunoprofiling Assay Kit.
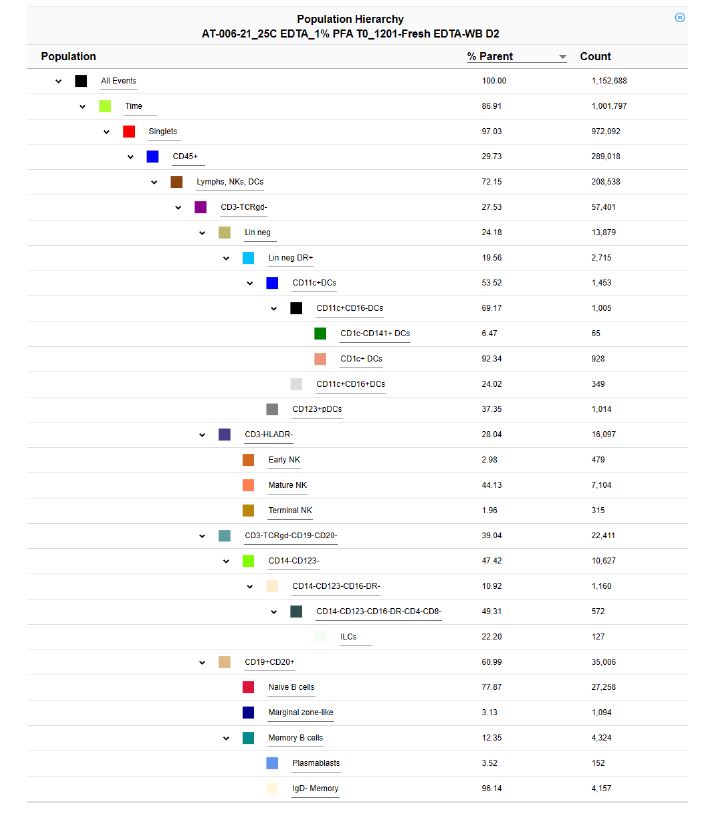
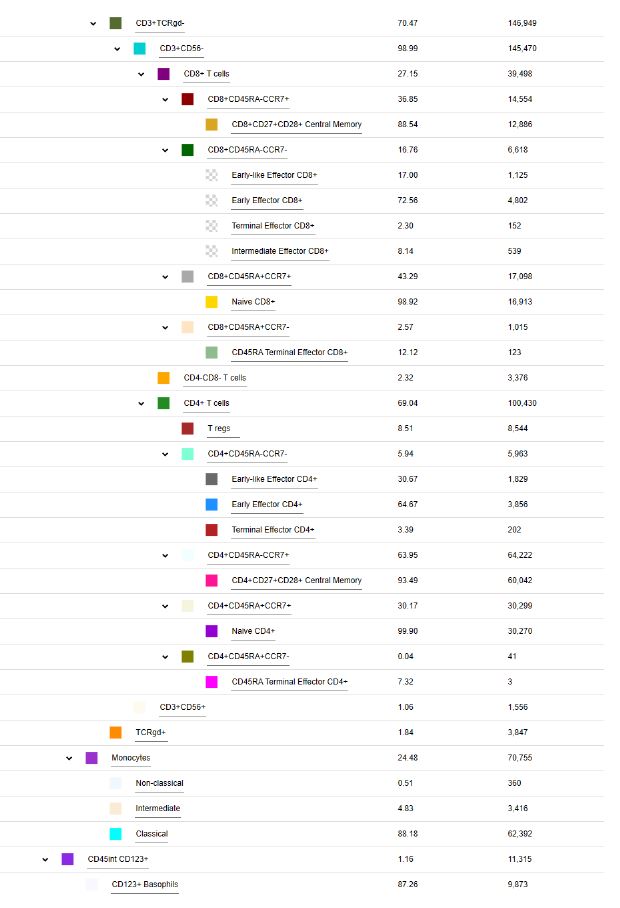
POPULATION HIERARCHY OF COLOR PANEL (as an example)
REFERENCES
1. Brestoff J. R. Full spectrum flow cytometry in the clinical laboratory. Int J. Lab. Hematol. 2023; 45(S2): 44-49. doi: 10.1111/ijlh.14098.
2. Jensen HA, Wnek R. Analytical performance of a 25-marker spectral cytometry immune monitoring assay in peripheral blood. Cytometry. 2021; 99: 180–193. https://doi.org/10.1002/cyto.a.24290.
3. https://cytekbio.com

.png)

.png)
