Multiple sclerosis (MS) is an autoimmune neurodegenerative disease that affects the central and peripheral nervous system [1].
The demyelination of neurons caused by MS disrupts the transmission of information within the brain and from the brain to body. The experience of a person with MS can change from day to day and the symptoms are also individual for each patient [2].
The main symptoms of MS include [1, 3]:
• Sensory loss;
• Paresthesia;
• Unilateral painful visual loss;
• Limb weakness;
• Facial weakness resembling idiopathic facial nerve palsy (Bell’s palsy);
• Visual blurring;
• Ataxia;
• Weariness;
• Fatigue;
• Pain;
• Bladder dysfunction;
• Cognitive dysfunction, usually mild, difficulty with multitasking;
• Sexual dysfunction;
• Lhermitte’s symptom (electric shock-like sensations evoked by neck flexion);
• Trigeminal or glossopharyngeal neuralgia, hemifacial spasm;
• Myokymia (rapid flashing contraction of the musculus pronator teres of the eye).
Uncommon symptoms of MS include [3]:
• Seizure;
• Dementia;
• Movement disorder.
MS is characterized by structural changes in the organs of the nervous system and one of the main manifestations is areas of inflammation and brain tissue damage – so-called “lesions”. The size, number and location of these lesions can determine the type and severity of the disease [3]. It is necessary to confirm the presence of several such lesions in more than one CNS region.
For this purpose, certain of the above symptoms should occur in the form of separate episodes lasting more than 24 h, and the interval between such episodes should be at least a month [1].
The main confirming method for the MS diagnosis is magnetic resonance imaging (MRI).
When the disease develops on MRI images of the patient can be detected:
• Cortex damage;
• A few different sized lesions;
• Lesions in motor tracts and Corpus Callosum;
• Reduction of gray matter volume and general brain volume;
• Reduction of spinal cord volume in cervical and thoracic cord areas.
The analysis of the cerebrospinal fluid (CSF) is additionally prescribed to diagnose MS along with an MRI scan. The main indicator of MS in the patient's CSF is the detection of oligoclonal bandings which is specific proinflammatory proteins. For additional testing, the level of immunoglobulins (IgG, IgM, IgA), total protein, glucose, and lactate is also checked [4].
According to the National Multiple Sclerosis Society for 2020 2.8 million people with MS are estimated to be living with the disease worldwide. Approximately 300 new cases of MS are recorded every day. According to the World Health Organization in 2021 Europe had the highest prevalence of MS with approximately 133 cases per 100,000 people. According to the National Health Service of Ukraine (NHSU) as of 2024 more than 32,000 patients with a confirmed diagnosis of MS were registered. In 2021 the NHSU recorded about 21,000 patients diagnosed with MS. That is over the previous three years an average of more than 3,500 cases per year were recorded in Ukraine.
However, despite the widespread prevalence of the disease, its diagnosis is quite complicated and time-consuming, as the CSF sampling (lumbar puncture) is a complex invasive procedure. Also, to MS diagnose several MRI scans with visible lesions of the nervous system are required at intervals of 6-12 months. What complicates establishing a diagnosis in the early stages of the disease.
The MS development is directly related to the destruction of the protective myelin sheath of the neuronal axon (Fig. 1B), which in turn contributes to the deterioration of nerve impulse transmission leading to the manifestation of clinical symptoms of the disease.


A. B.
Figure 1. A – normal neuron; B – MS mediated neuronal demyelination process.
Neurofilament light chain (NfL) are components of the axon cytoskeleton. They are responsible for maintaining normal neuronal morphology and nerve fiber elasticity, preventing their rupture and promoting radial axonal growth. A large number of studies have shown that extracellular NfL levels change more significantly with the onset and development of various diseases of the nervous system indicating that NfL is a molecular marker of neuronal damage and may act as a marker of various neurological disorders [5].
During demyelination process NfL release significantly increases which enters the CSF and human peripheral blood, due to its ability to cross the blood-brain barrier. Therefore, most modern studies aimed at determining NfL for the diagnosis of neurodegenerative disorders use enzyme-linked immunosorbent assay (ELISA).
Based on clinical trials, Siemens Healthineers has developed an assay kit for the Atellica IM/Atellica CI analyzers for quantitative determination of NfL in human serum or EDTA plasma.
This test system has certain advantages compared to the ELISA method:
• Often, the ELISA sensitivity is insufficient for the detection of NfL in human serum, due to very low concentrations of the analyte, as the ratio of NfL concentration in serum to CSF can be approximately 1:40;
• The Atellica IM/Atellica CI analyzers are a fully automatic closed system that minimizes errors due to human factor, as ELISA is a completely manual technique;
• Better reagents stability due to their permanent onboard location with a cooling system and minimal contact with the external environment;
• The Atellica IM/Atellica CI assay takes 51 minutes, whereas ELISA can take up to 4 hours.
The Atellica CI is a biochemical (CH) and immunoassay (IM) analyzer that also has an electrolyte sensor.
The CH and IM units have no shared elements, which speeds up results throughput. The Atellica CI is capable of measuring 600 CH, 120 IM, and 400 electrolytes per hour. It has 70 CH and 20 IM reagent position on board, and an integrated multisensor technology for measuring Na+, K+, Cl-.
The analyzer accepts samples of serum, plasma, amniotic fluid, urine, whole blood (for specific assays), and CSF. It also has integrated software with intelligent monitoring of consumables, and is capable of detecting clots, air bubbles, to check lipemia, hemolysis, and icterus index in samples.
The Atellica IM is an immunoassay unit as part of the Atellica Solution analyzer. The Atellica Solution is an assembly line of several units (CH and/or IM) and a sample loading station that has a 360° 3D scanner for better barcode reading. It has a patented transport line for fast sample delivery between units and the ability to prioritize samples. The throughput of the CH unit is 1200 tests per hour and 600 electrolytes per hour. The IM unit is capable of measuring 440 tests per hour. The CH unit can simultaneously accommodate 67 reagent packs and 3 positions for electrolytes. The IM unit can accommodate 42 reagent kits on board. The Atellica Solution analyzer can include up to 10 units.
Atellica IM/Atellica CI performs two-phase acridine ester (AE) based immunochemiluminescence with the addition of paramagnetic microparticles (PM) (Fig. 2). The reagent for the NfL detection contains AE labeled monoclonal mouse antibodies, which interact with NfL from the patient's sample (liquid phase). After sample incubation, biotinylated mouse monoclonal antibodies to NfL bound to streptavidin-coated PMs are added to the solution (solid phase). The formed complexes are attracted to the magnet, and unbound elements are removed during washing step. Next step is adding an acidic and alkaline reagent to initiate luminescence.
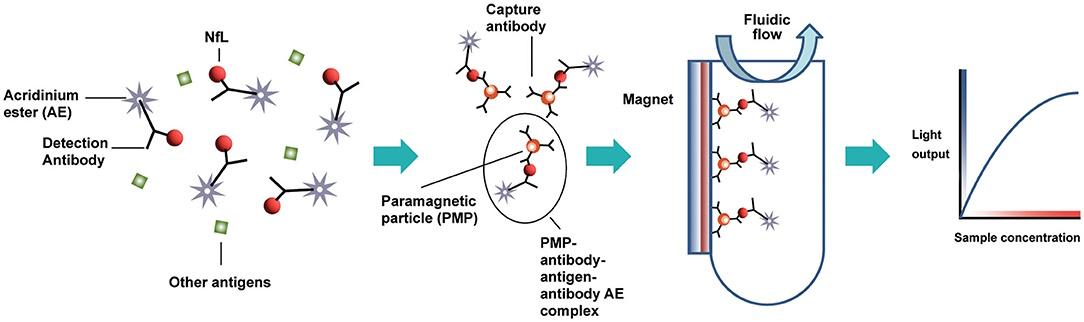
Figure 2. NfL detection method principle in test sample on the Atellica IM/ Atellica CI analyzer [6].
The high-affinity test system for NfL quantitative detection allows to determine the presence of NfL in human plasma and serum in concentrations of 3.0-300 pg/ml. The main advantage of this method is that due to high sensitivity of the method the patient can avoid lumbar puncture, which significantly simplifies the diagnostic process. The NfL assay efficiency on Atellica IM analyzer was evaluated using samples from adult patients with relapsing MS (aged 18-55 years) in a pharmaceutical clinical trial. As a result, an NfL levels increase in the patient’s serum with relapsing MS was confirmed.

Fig. 3. Reagent kit for the determination of NfL.
The Atellica IM/Atellica CI assay kit includes 1 package of primary reagent with AE and PMs for 100 tests (Fig. 3A), vials with calibrators low and high level one of each and calibrator assigned value cards. Quality control three levels with assigned value cards and diluent for diluting samples with exceeding the measuring capacity of the test system concentration (>300 pg/ml), and instructions for the kit which includes a description of the test, reagent storage conditions, and preparation and storage of the samples are not included in the kit.
References
1. Stephen L.H., Bruce A.C.C. Treatment of Multiple Sclerosis: A Review. 2020
2. https://www.nationalmssociety.org/
3. https://mymsaa.org/ms-information/overview/process-symptoms/
4. Brett S.T., Benjamin K.T.T., Joshua L.B. Multiple sclerosis: Diagnosis, disease-modifying therapy and prognosis. 2022
5. Xiaoting Z., Huixian W., Li L. Neurofilament Light Chain: A Candidate Biomarker of Perioperative Stroke. 2022
6. Lee S., Plavina T., Singh C.M. Development of a Highly Sensitive Neurofilament Light Chain Assay on an Automated Immunoassay Platform. 2022
7. https://www.bioworld.com/
.jpg)
.png)

.png)
