- Home/
- Products/
- Molecular diagnostics/
- PCR/
- PCR reagent kits/
- TRUPCR® ALK Fusion Detection Kit
- IVD
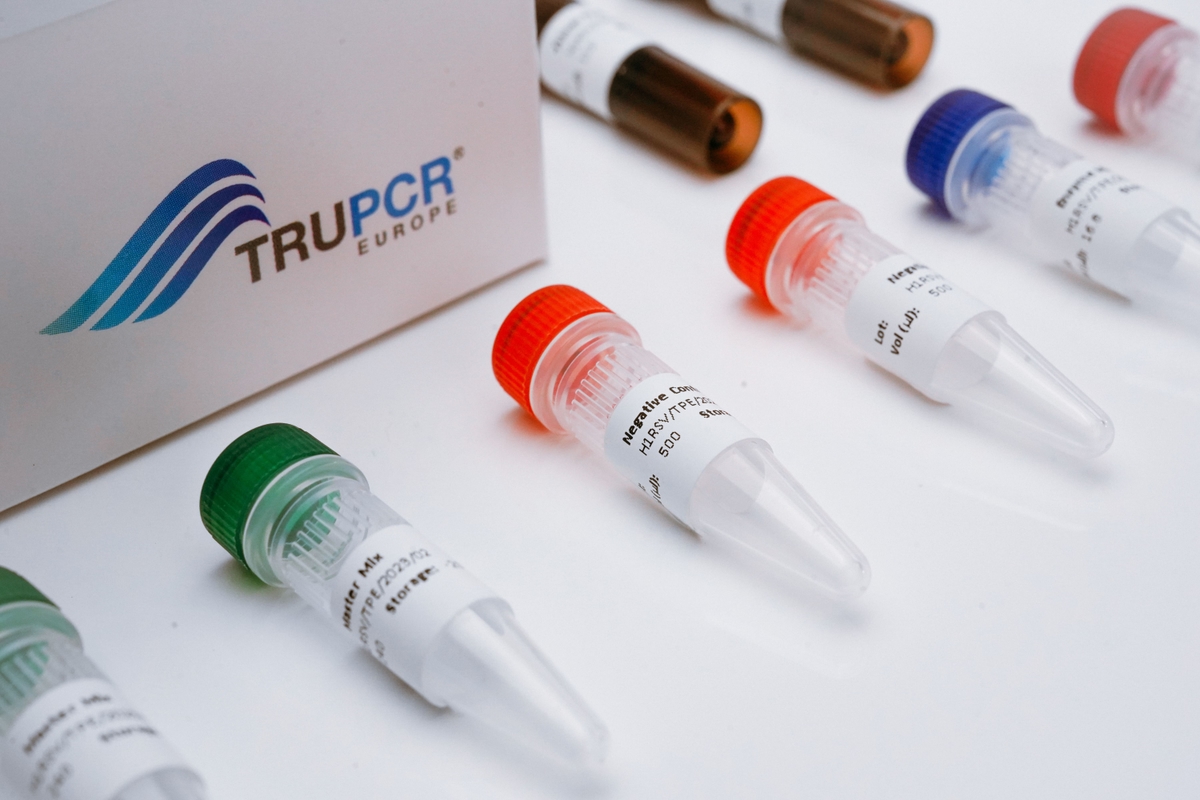
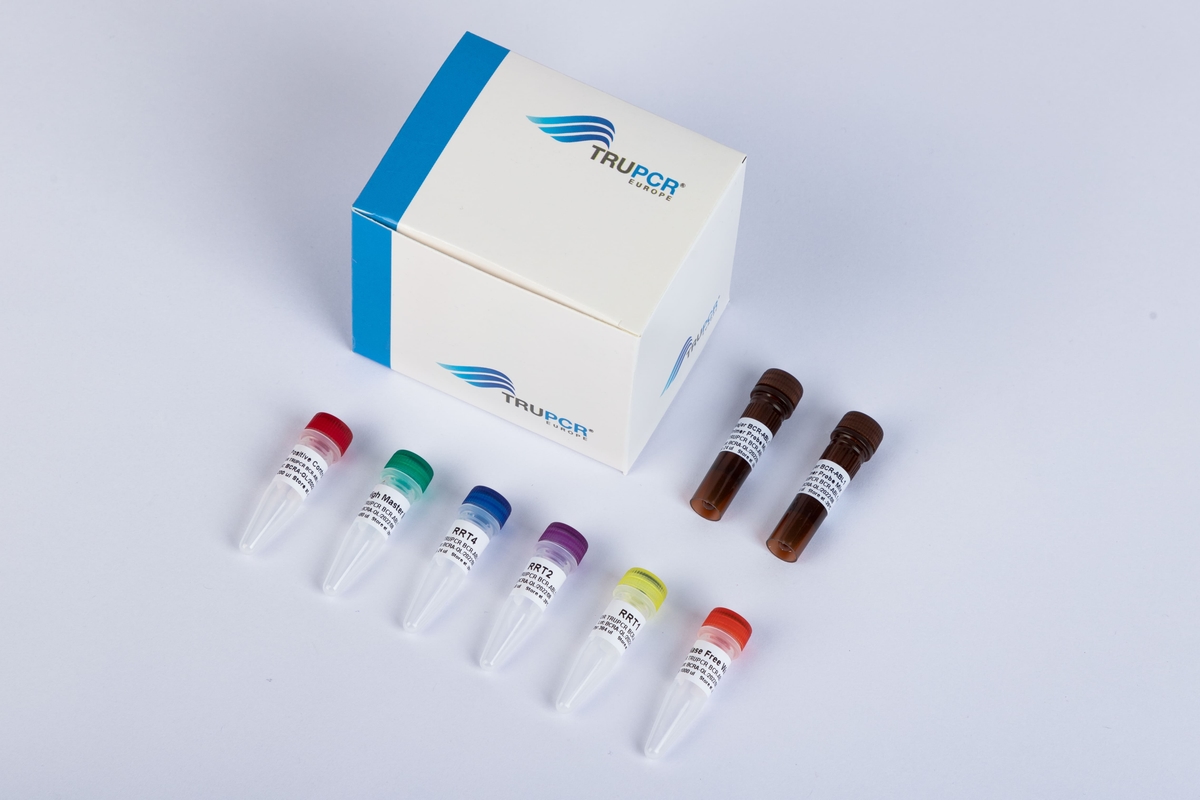
TRUPCR® ALK Fusion Detection Kit
| Manufacturer: | TRUPCR |
|---|---|
| Country: | United Kingdom |
Specialist consultation
+38 (067) 880 44 83Details
The TRUPCR® ALK Fusion Detection Kit is intended for the qualitative detection of 29 EML4-ALK fusions in human NSCLC formalin-fixed paraffin-embedded (FFPE) tissue samples using Real-time PCR.
The human ABL1 gene serves as an internal positive control for human nucleic acid, also included in this kit.
Key Features:
• The kit allows the detection of 29 EML4-ALK fusions
• All controls and assay reagents included in the kit
• Highly sensitive with a LOD of up to 10 copies
• Compatible with various Real Time PCR instruments
• Easy-to-use, rapid, reliable, comprehensive and cost-effective test
3B1343 – 24 tests
3B1344 – 48 tests
Characteristics
| Analysis type | Qualitative |
|---|---|
| Number of tests | 24 / 48 |
| Instruments | ▪ Applied Biosystems™ 7500, ▪ StepOne and StepOnePlus, ▪ QuantStudio® 3, 5 та 12, ▪ Rotor-Gene Q, ▪ Bio-Rad CFX96, CFX384, ▪ AriaMx Real-Time PCR, ▪ Roche - LightCycler® 480 -II, ▪ Line gene K real time PCR |
| Reporting units | DNA copies/ml eluate |
| Regulatory status | CE IVD |
|---|---|
| Required detection channels | HEX/VIC, Tex Red/ ROX |
| Storage | at -20°C until ready to use and keep on ice during use |
Service and Warranty
We provide premium service and maintenance for every device and consumable:
• Comprehensive maintenance of medical equipment
• Installations, commissioning
• Warranty and post-warranty service
• Instruction, training and consultations for your team
More details on the SERVICE page
| Contact phone | +38 (067) 133 05 52 |
|---|---|
| E-mail: | service@empirica.healthcare |
.jpg)
.jpg)